
Gut Microbiome Therapeutics Market Growth 2024–2034 | CAGR 23.2%
Global Gut Microbiome Therapeutics Market Size, Share, Analysis Report ByType (Fecal Microbiota Transplantation, Microbiome Drugs) , Application (Diabetes, Crohn’s Disease, Clostridium Difficile Infection , Multiple Sclerosis, Rheumatoid Arthritis, Others), Product (Prebiotics, Probiotics), Region and Key Players - Industry Segment Overview, Market Dynamics, Competitive Strategies, Trends and Forecast 2025-2034
Report Overview:
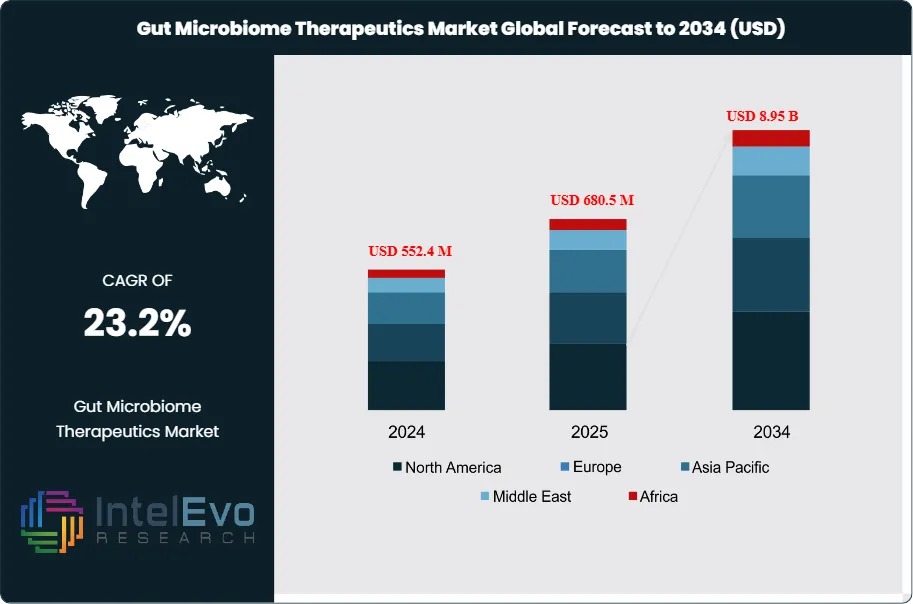
The Global Gut Microbiome Therapeutics Market size is projected to reach approximately USD 8.95 billion by 2034, up from USD 552.4 million in 2024, growing at a CAGR of 23.2% during the forecast period from 2024 to 2034. Growing awareness of the gut microbiome’s role in overall health, coupled with advancements in microbiome-based drug development, is driving rapid adoption across pharmaceutical and biotech industries. With increasing investments, clinical trials, and partnerships, this market is poised to reshape personalized medicine and open new frontiers in digestive, metabolic, and neurological healthcare solutions.
Get More Information about this report -
Request Free Sample ReportThe Gut Microbiome Therapeutics Market focuses on products designed to modulate gut microbiota to enhance health outcomes. This market includes fecal microbiota transplantation (FMT), probiotics, prebiotics, and microbiome-based pharmaceuticals. The dynamics of this market are changing as awareness of the gut's critical role in overall health increases. In 2024, the market is valued at approximately USD $552.4 million, reflecting substantial investments in microbiome research and innovation. The growing prevalence of gastrointestinal disorders, diabetes, and obesity drives demand for microbiome therapies, indicating a promising growth trajectory for the sector.
Several factors are contributing to the robust growth of the gut microbiome therapeutics market. Key growth drivers include the increasing acknowledgment of the gut microbiome's influence on a wide array of health conditions, such as autoimmune diseases and metabolic disorders. The rising incidence of Clostridium difficile infections, along with a growing interest in personalized medicine, further propels market growth. Technological advancements in biotechnology and genomics facilitate the development of innovative microbiome-based products. Additionally, improved regulatory support and funding for microbiome research contribute to the positive outlook of the market. The gut microbiome therapeutics market is projected to grow at a CAGR of 23.6% from 2024 to 2034, reaching USD $8.59 billion by the end of the forecast period.
North America is expected to lead the gut microbiome therapeutics market, holding approximately 40% of the total market share by 2037. This dominance is attributed to advanced healthcare infrastructure, increased healthcare spending, and a strong focus on research and development. Europe is anticipated to capture around 29% of the market, supported by favorable regulatory environments and a high prevalence of gastrointestinal disorders. In contrast, the Asia-Pacific region is likely to exhibit significant growth due to rising awareness of gut health and improved healthcare access, making it a promising market for future investments.
The COVID-19 pandemic has significantly impacted the gut microbiome therapeutics market. Although initial disruptions in clinical trials and supply chains presented challenges, the pandemic heightened awareness of gut health and immunity. The increased focus on preventive healthcare has led to a surge in interest in microbiome-based products as potential solutions for maintaining health during and after the pandemic. This shift in consumer behavior is expected to sustain the market's momentum in the post-pandemic landscape, further solidifying its growth trajectory.

Key Takeaways:
- Market Growth: The gut microbiome therapeutics market is expected to reach USD $8.95 billion by 2034, growing at a robust CAGR of 23.2%, indicating strong market expansion.
- Type Segment Analysis: Fecal Microbiota Transplantation (FMT) is a leading segment, primarily used for treating Clostridium difficile infections. It showcases significant clinical effectiveness, driving its adoption and market share within gut microbiome therapeutics.
- Application Segment Analysis: Diabetes is anticipated to be a prominent application, as emerging research highlights the gut microbiome's role in metabolic health. This focus positions diabetes-related products for considerable market demand and growth.
- Driver: Increasing recognition of the gut microbiome's influence on health, particularly in managing gastrointestinal disorders and autoimmune diseases, is a key driver of market growth, along with advancements in personalized medicine.
- Restraint: High production costs and regulatory hurdles associated with developing microbiome-based therapies can limit market accessibility and slow down the introduction of new products in the market.
- Opportunity: The expanding demand for personalized medicine and the increasing prevalence of gut-related health issues in developing regions, particularly in Asia-Pacific, present significant growth opportunities for industry players.
- Trend: The trend towards preventive healthcare is rising, with consumers increasingly seeking microbiome-based solutions to enhance overall health and immunity.
- Regional Analysis: North America is projected to dominate the market, driven by advanced healthcare infrastructure and significant investments in microbiome research, while the Asia-Pacific region shows promising growth potential due to rising awareness of gut health.
By Therapy Type:
The therapy type segment of the Global Gut Microbiome Therapeutics Market includes microbiome-based drugs, probiotics and prebiotics, live biotherapeutics, postbiotics and synbiotics, and fecal microbiota transplants (FMT). Microbiome-based drugs and live biotherapeutics are gaining traction because they can directly modify microbial balance and improve patient outcomes. Probiotics and prebiotics are still widely used for preventive healthcare, while postbiotics and synbiotics show promising therapeutic potential. FMT remains effective for recurrent infections like C. difficile. Together, these therapies create a diverse landscape that meets both preventive and curative healthcare needs, playing an important role in market growth and innovation.
By Indication:
The market by indication shows the broad clinical use of gut microbiome therapeutics. Key areas include gastrointestinal disorders such as IBD, IBS, Crohn’s disease, and ulcerative colitis, where microbial imbalance is critical. Therapies are also being studied for metabolic disorders like diabetes and obesity, as well as infectious diseases such as recurrent C. difficile. Neurological disorders, including Parkinson’s, autism, and depression, are getting more attention as research on the gut-brain connection grows. Furthermore, oncology applications are emerging, where microbiome adjustment improves cancer treatment effectiveness. This wide range highlights the transformative potential of microbiome-based therapies across many therapeutic areas.
By Route of Administration:
Segmentation by route of administration shows how delivery methods are vital for ensuring treatment effectiveness. Oral delivery is the most common and preferred method for probiotics, prebiotics, and microbiome-based drugs due to its ease of use and patient compliance. Intravenous (IV) delivery is used for advanced bio-therapeutics that need to reach systemic circulation, while rectal administration is important in FMT procedures. New delivery systems, including encapsulated microbes and engineered formulations, are creating new possibilities by improving stability, targeted delivery, and bioavailability. The variety of administration routes is key to addressing individual patient needs and maximizing clinical success in microbiome therapeutics.
By Distribution Channel:
The distribution channel segment emphasizes the importance of different access points for gut microbiome therapeutics. Hospitals and clinics lead the way because they are essential for prescribing advanced therapies and conducting FMT procedures. Specialty pharmacies are an important channel for microbiome-based drugs and bio-therapeutics, providing tailored services for complex prescriptions. Online pharmacies are quickly becoming a growth area, boosted by increasing consumer interest in probiotics, prebiotics, and over-the-counter microbiome supplements. Research and academic institutes also play a significant role, especially in early-stage product trials and experimental therapies. This varied distribution network ensures that microbiome solutions are available in both clinical and consumer settings.
By End User:
The end user segment highlights the groups driving growth in the gut microbiome therapeutics market. Pharmaceutical companies are investing heavily in drug development, focusing on regulatory approvals and commercialization. Biotech companies lead innovation, exploring next-generation therapies and personalized solutions. Research organizations and academic institutes are crucial for advancing scientific understanding and clinical validation of microbiome-based therapies. Additionally, Contract Research Organizations (CROs) are vital for supporting clinical trials, regulatory work, and scalability. Together, these end users create an integrated ecosystem that promotes innovation, speeds up product development, and encourages global market adoption.
Region Analysis:
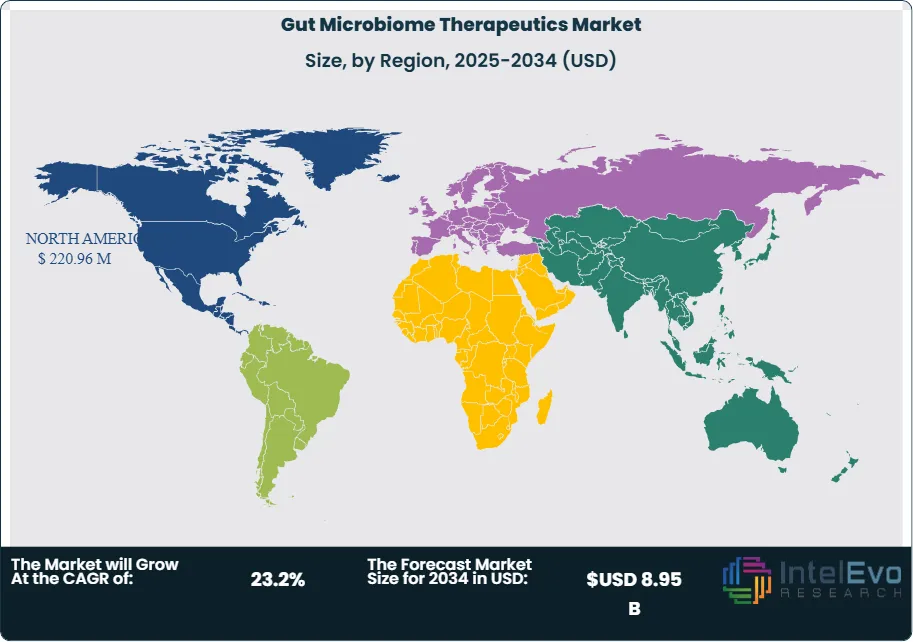
North America leads with 40% Market Share in the Gut Microbiome Therapeutics Market: North America holds the largest market share in the gut microbiome therapeutics market, accounting for approximately 40% of the total revenue. This dominance is primarily driven by advanced healthcare infrastructure, significant investments in microbiome research, and a high prevalence of gastrointestinal disorders. The presence of key players and innovative biotechnology firms in the region enhances product development and accelerates the introduction of new therapies. Additionally, the increasing awareness among consumers regarding gut health and its correlation with overall well-being supports market growth. Regulatory bodies in North America have also established supportive frameworks for microbiome-related therapies, further contributing to the market's expansion. The strong focus on research and development in universities and research institutions reinforces the region's leadership in this field.
Asia-Pacific is recognized as the fastest-growing region in the gut microbiome therapeutics market, with a projected CAGR exceeding 25% over the next decade. Several factors contribute to this rapid growth, including rising consumer awareness of the importance of gut health, increased healthcare expenditure, and the growing prevalence of gut-related health issues. The expanding middle class in countries like China and India is driving demand for advanced healthcare solutions, including microbiome therapies. Furthermore, ongoing research initiatives and collaborations between academic institutions and biotech companies in the region are fostering innovation and product development. Other regions, such as Europe and Latin America, are also witnessing steady growth, driven by increasing investments in microbiome research and a growing emphasis on preventive healthcare. The Middle East and Africa are gradually emerging, focusing on health awareness campaigns and improving healthcare infrastructure, contributing to overall market expansion.
Get More Information about this report -
Request Free Sample ReportKey Market Segment
By Therapy Type
- Microbiome-based Drugs
- Probiotics & Prebiotics
- Live Biotherapeutics
- Postbiotics & Synbiotics
- Fecal Microbiota Transplant (FMT)
By Indication
- Gastrointestinal Disorders (IBD, IBS, Crohn’s Disease, Ulcerative Colitis)
- Metabolic Disorders (Diabetes, Obesity)
- Infectious Diseases (C. difficile infection, others)
- Neurological Disorders (Parkinson’s, Autism, Depression)
- Oncology Applications
- Other Indications
By Route of Administration
- Oral
- Intravenous (IV)
- Rectal
- Other Delivery Methods
By Distribution Channel
- Hospitals & Clinics
- Specialty Pharmacies
- Online Pharmacies
- Research & Academic Institutes
By End User
- Pharmaceutical Companies
- Biotech Companies
- Research Organizations
- Contract Research Organizations (CROs)
By Region
- North America
- Latin America
- East Asia And Pacific
- Sea And South Asia
- Eastern Europe
- Western Europe
- Middle East & Africa
| Report Attribute | Details |
| Market size (2025) | USD 680.5 M |
| Forecast Revenue (2034) | USD 8.95 B |
| CAGR (2025-2034) | 23.2% |
| Historical data | 2018-2023 |
| Base Year For Estimation | 2024 |
| Forecast Period | 2025-2034 |
| Report coverage | Revenue Forecast, Competitive Landscape, Market Dynamics, Growth Factors, Trends and Recent Developments |
| Segments covered | By Therapy Type (Microbiome-based Drugs, Probiotics & Prebiotics, Live Biotherapeutics, Postbiotics & Synbiotics, Fecal Microbiota Transplant (FMT)), By Indication (Gastrointestinal Disorders (IBD, IBS, Crohn’s Disease, Ulcerative Colitis), Metabolic Disorders (Diabetes, Obesity), Infectious Diseases (C. difficile infection, others), Neurological Disorders (Parkinson’s, Autism, Depression), Oncology Applications, Other Indications), By Route of Administration (Oral, Intravenous (IV), Rectal, Other Delivery Methods), By Distribution Channel (Hospitals & Clinics, Specialty Pharmacies, Online Pharmacies, Research & Academic Institutes), By End User (Pharmaceutical Companies, Biotech Companies, Research Organizations, Contract Research Organizations (CROs)) |
| Research Methodology |
|
| Regional scope |
|
| Competitive Landscape | Seres Therapeutics, Finch Therapeutics, MaaT Pharma, Enterome, Evelo Biosciences, Rebiotix (A Ferring Company), Viome, Second Genome, AnimalBiome, Synlogic, 4D Pharma, ClostraBio, Probi AB, TargEDys, Genomatica, Medosome Biotec, Kintai Therapeutics, Microbiome Therapeutics, Adiso Therapeutics, BiomeBank |
| Customization Scope | Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. |
| Pricing and Purchase Options | Avail customized purchase options to meet your exact research needs. We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF). |
Select Licence Type
Connect with our sales team
Gut Microbiome Therapeutics Market
Published Date : 17 Dec 2024 | Formats :Why IntelEvoResearch
100%
Customer
Satisfaction
24x7+
Availability - we are always
there when you need us
200+
Fortune 50 Companies trust
IntelEvoResearch
80%
of our reports are exclusive
and first in the industry
100%
more data
and analysis
1000+
reports published
till date







